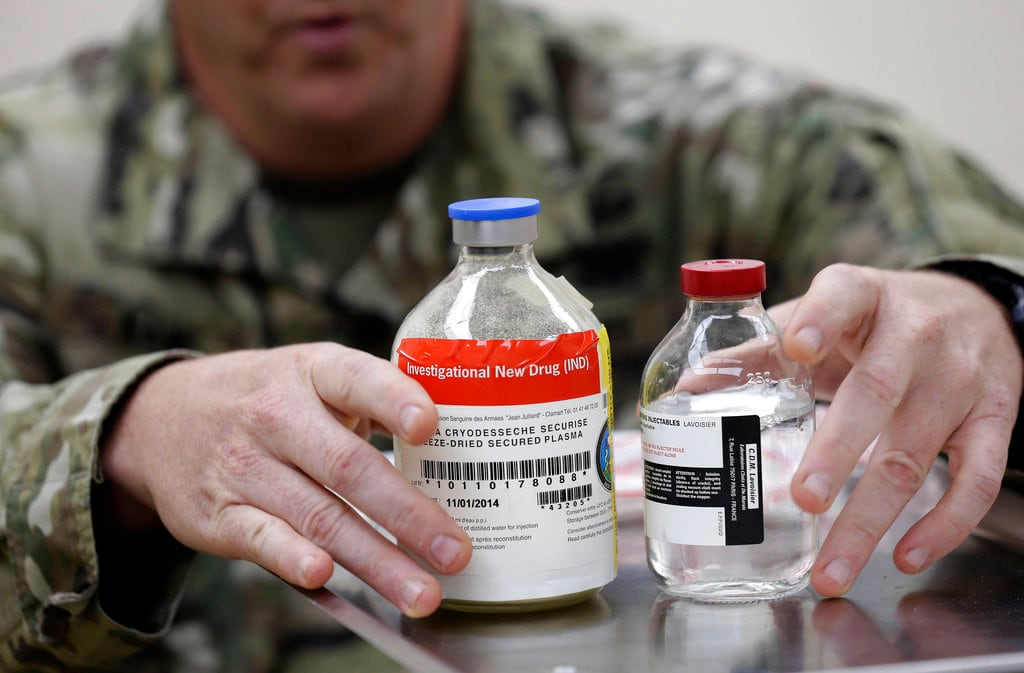
After a year full of discussion and disagreement over whether freeze-dried blood plasma would be accessible for use by the Department of Defense, the FDA has approved the use of the potentially life-saving product to treat injuries to U.S. troops, according to The Hill.
Plasma plays an important role in the human body, and it has clotting properties that can stop bleeding, especially in life or death circumstances on the battlefield.
Freeze-dried plasma is easier to transport than the liquid form of plasma, especially in an environment like the battlefield. Plasma needs to be kept cold, so freeze-drying the solution avoids the need to keep it at an optimal temperature while in a medical kit in combat. The product has a shelf life of about two years.
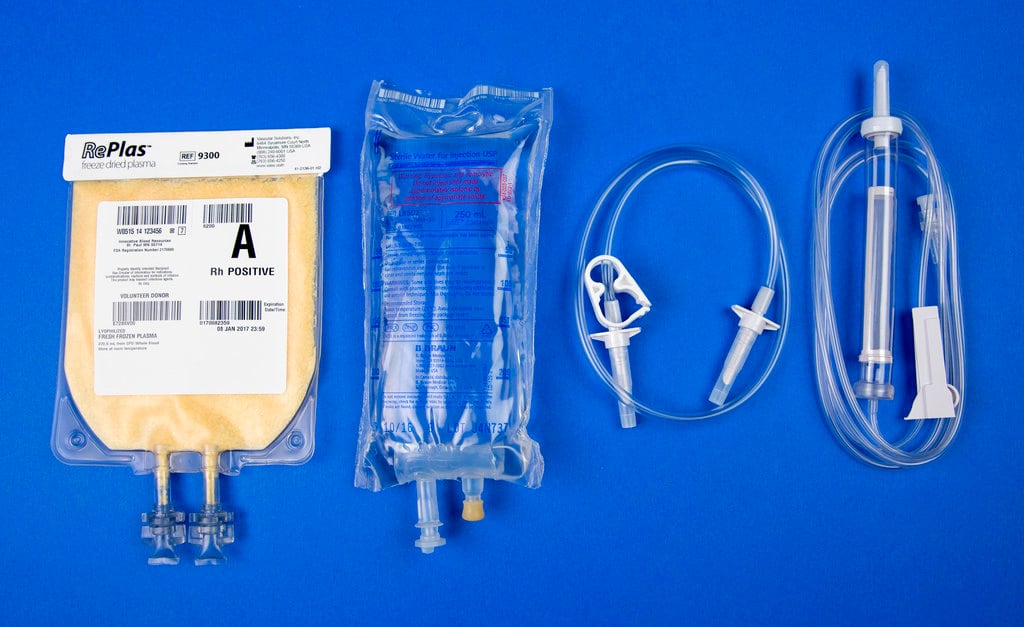
The freeze-dried version of plasma is a powder that only needs to be mixed with sterile water before it can be injected into the patient. The resulting product prevents soldiers from needing to carry actual plasma in coolers or other temperature-regulating storage containers that can be cumbersome and bulky,
The FDA’s approval of the product for use comes nearly a year after House Armed Services Committee Chairman Mac Thornberry (R-Texas) tried to allow DoD to approve the product for emergency use in combat. according to The Hill.
The 2018 National Defense Authorization Act would have given DoD that power, but the necessary language was removed from the NDAA after the FDA and its congressional supporters fought back on the change. A new bill was passed that instead authorizes DoD to request the quickening of the FDA’s review of medical products.
The FDA and DoD began working together at the beginning of the year in an effort to fast-track the use of the potentially life saving product, according to the Washington Examiner.
RELATED

As of January, the US was using the French product until Teleflex Inc. can produce the product for the armed services. France has had approval from their equivalent of the FDA for 24 years.
While the FDA approval of the military use of freeze-dried blood plasma is new, the use of it is not. In March 2017, Lt. Col. Rebecca Carter, then chief of Air Force Special Operation Command’s medical modernization, said that the U.S. used freeze-dried plasma in WWII, but stopped when the use of the product was linked to hepatitis outbreaks because of the unreliable methods of donor screening at the time.
Noah Nash is a rising senior at Kenyon College in Gambier, Ohio. At school, he is the editor in chief of the Collegian Magazine and the digital director of the Collegian, Kenyon's newspaper.