Sixteen sites around the world are preparing to administer the military’s first doses of COVID-19 vaccine to health care workers and other essential personnel, as soon as the Food and Drug administration approves the two-dose Pfizer regimen for emergency use.
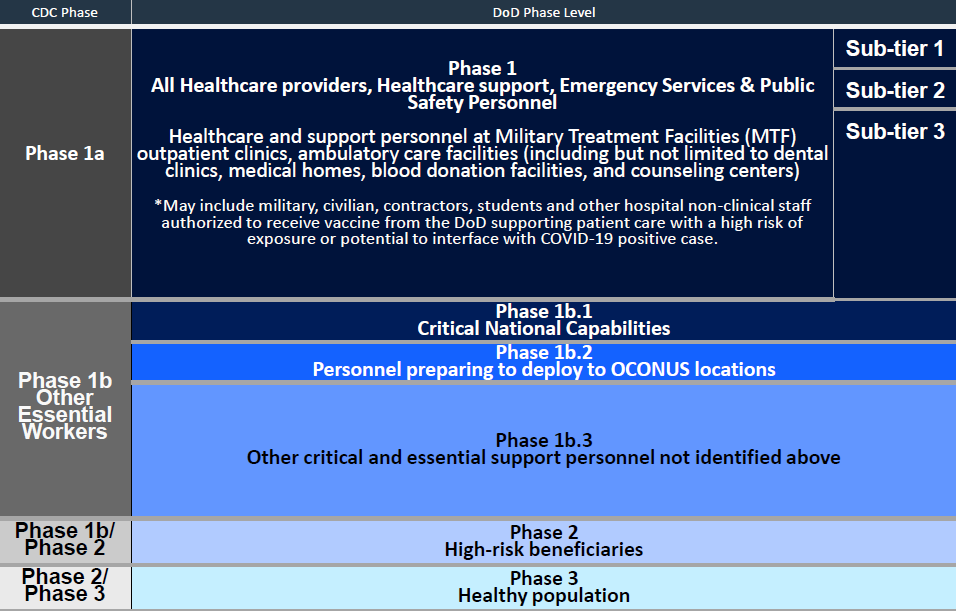
Using the Centers for Disease Control and Prevention’s plan for vaccination priorities, the Defense Department will vaccinate uniformed and civilian health care workers first, before working their way down through a plan that later includes critical national security units, troops preparing to deploy outside the country and so on.
“We expect to have shots in arms of DoD personnel within 20 to 48 hours from the time the [FDA advisory panel] issues its final recommendation,” Thomas McCaffery, the defense under secretary for health affairs, told reporters on Wednesday.
DoD expects to get 44,000 initial doses of the vaccine, which will initially go to 13 U.S.-based sites, and three overseas, where officials have determined there are both the conditions to store them properly as well as enough highest-priority personnel to make use of them.
Those could go out as soon as next week, McCaffery said, as the expectation is that FDA will meet and render its decision sometime this week.
Once the supply chain gets rolling, vaccines will be sent force-wide.
Nearly all of those initial doses will go to health care workers and other medical or counseling staff, including reservists and Guardsmen.
A “very, very, very limited” number of them will go to senior department officials, Lt. Gen. Ronald Place, head of the Defense Health Agency, said.
Those may include acting Defense Secretary Chris Miller, Deputy Defense Secretary David Norquist, Chairman of the Joint Chiefs Army Gen. Mark Milley and Vice Chairman of the Joint Chiefs Air Force Gen. John Hyten, Pentagon spokesman Jonathan Hoffman said, adding that they have been encouraged to get their vaccines administered publicly.
Senior leaders will be doing a good amount of public outreach for the vaccine, because unlike the many other mandatory injections for service members, this one is going to be voluntary for a while.
“Our advice to everyone would be to take the vaccine, just based on risk,” Place said, place said, adding that the initial feedback on the safety and efficacy of the vaccines is very good.
The possible side effects will sound familiar to anyone who’s had a vaccine before, including a sore arm and potentially a fever.
DoD is also recommending that COVID-19 survivors also get the vaccine, McCaffery said, as research is inconclusive about how effective antibodies are and how long they last.
As of Wednesday, 86,007 troops, 22,553 DoD civilians, 13,202 dependents and 7,838 DoD contractors have tested positive for the virus and 13 service members have died from COVID complications.
RELATED

Keeping the vaccine voluntary initially is consistent with the emergency use authorization. Once FDA has signed a full approval, Place said, the department will take a look at making it mandatory.
Once medical and clinical staff are vaccinated, high-level national security personnel like nuclear submarine sailors, Air Force bomber crews and special operations counter-terror units will follow, and deploying troops will follow them, much like the current COVID-19 testing protocol DoD implemented in April.
The next groups include other support personnel, like military police, firefighters and others who are not able to telework or minimize their contact with other people,
Once those groups are squared away, according to the plan, DoD will set about vaccinating high-risk dependents and other civilian personnel, based on risk factors like age and pre-existing conditions.
The final phase will include healthy personnel not considered “on the front lines,” but McCaffery couldn’t say when he expects all volunteers will be vaccinated.
There are several factors that will affect that, including whether and when a second vaccine candidate, from Moderna, gets its emergency use authorization and how quickly those vaccines can roll out. Each vaccine also requires a booster, adding amount a month onto the timeline after everyone has received an initial dose.
Meanwhile, DoD will continue its testing and quarantine policies, with some updates forthcoming.
In light of the CDC reducing its quarantine recommendations from 14 days to 5 to 10, depending on whether an exposed person has taken a COVID-19 test, DoD is considering reducing its 14-day quarantines around travel or deployments, McCaffery said, though a policy hasn’t been finalized.
Meghann Myers is the Pentagon bureau chief at Military Times. She covers operations, policy, personnel, leadership and other issues affecting service members.